BEYOND MERE NOVELTY
The invention of the smartphone and the development of its peripherals have promoted the advancement of wearable devices. Technological innovations and applications have enabled the creation of numerous wearable devices. It seemed as though wearable devices were destined to become an integral part of our lives. However, although most wearable devices initially stimulated public curiosity, they have not been consistently adopted. A large-scale investigation revealed that the abandonment rate of wearable devices dropped sharply during the first six months, and after 15 months, half of the users had abandoned their devices (Endeavour Partners, 2014). Another study found that only 20% of users continued using their devices after six months (Gartner, 2016).
Adopting wearable devices only to abandon them is a significant waste of resources. From a consumer’s perspective, this cycle represents not only a personal misallocation of financial and temporal resources but also contributes to a global issue of resource wastage. Initially, consumers invest considerable sums of money in acquiring these cutting-edge devices, drawn by the promise of enhanced daily living through technology. This financial investment is often justified by the expectation of receiving long-term benefits, such as improved health monitoring, better communication, or enhanced productivity. Furthermore, consumers dedicate substantial time to selecting the right device, learning its functionalities, and integrating it into their daily routines. However, when these devices fail to meet expectations or are rendered obsolete due to rapid technological advancements, they are quickly abandoned.
This cycle reflects not only a loss of personal investment and effort but also exacerbates the problem of electronic waste. From an environmental perspective, the production, use, and disposal of wearable devices consume valuable resources, including rare minerals and energy, while contributing to the growing accumulation of e-waste (Verdict, 2021). This e-waste can have harmful environmental impacts, and if not properly disposed of or recycled, it can lead to severe environmental problems (EWCra, 2024). Therefore, for the sustainable development of wearable technology, environmentally friendly design and efficient resource management are essential (Gurova et al., 2020).
The author aims to uncover the fundamental reasons behind this trend, examining the interplay between technological expectations and reality. Beyond identifying these issues, the focus will shift toward practical solutions to make wearable devices in specialized fields. This includes designing devices centered on value, emphasizing practical applications over novelty. Such analysis is vital for technology enthusiasts, developers, and consumers interested in wearable devices, as it provides valuable insights into the future of this technology.
WHY DO PEOPLE ABANDON WEARABLE DEVICES?
People adapt to the existing world around them. Even if new technologies are developed to make life slightly more convenient and efficient, they cannot create a ripple effect unless they provide clear advantages. Thus, when innovative technologies emerge, the tangible benefits they offer are more critical than the innovation itself.
Most wearable devices are designed to enhance the healthcare of the general population. While some may believe that acquiring and analyzing human-derived real-world data will transform lifestyles and improve health outcomes, significant hurdles remain (Gao et al., 2015; Lunney et al., 2016; Piwek et al., 2016). Consumers did not change their behavior as device inventors and retailers anticipated (Gao et al., 2015). In other words, they felt that wearable devices failed to provide sufficient benefits to warrant changes in their behavioral patterns.
Changing ingrained behaviors and habits presents an immense challenge, even for individuals managing chronic illnesses that necessitate lifestyle modifications for effective treatment. Type 2 diabetes exemplifies a chronic condition requiring substantial lifestyle changes, such as dietary adjustments, increased physical activity, and adherence to prescribed medication regimens. Despite the seriousness of this condition and clinicians’ repeated emphasis on the importance of these changes, a concerning reality persists: few patients strictly adhere to these recommendations.
A study of individuals with Type 2 diabetes revealed an alarming statistic, 58.1% of participants reported never implementing any lifestyle changes (Adu et al., 2019). This highlights the profound difficulty of altering entrenched behaviors, even when health is at stake. If those already facing chronic conditions struggle to initiate and sustain necessary changes, it stands to reason that individuals without diagnosed medical issues would be even less motivated to adopt healthier habits.
Consequently, dedicating resources to promoting lifestyle changes among the general, healthy population may not be the most effective approach. A more pragmatic strategy would be to focus on identifying applications of wearable devices within the medical care sector. This domain holds immense promise for leveraging technology to enhance patient outcomes and facilitate behavior change.
The medical process is traditionally divided into three phases: diagnosis, treatment, and follow-up monitoring. For wearable devices to reach their full potential in improving public health, they must demonstrate their ability to contribute meaningfully to one or more of these critical stages (Dunn et al., 2018). By seamlessly integrating into established medical protocols, wearable technologies could revolutionize healthcare delivery, monitoring, and maintenance. Ultimately, this would empower individuals to take a more active role in managing their well-being.
CLASSIFICATION AND SPECIFICATIONS
The author categorizes the utility of wearable devices into several sectors (Fig. 1). Wearable devices, excluding those classified within the realm of digital therapeutics, are versatile tools widely utilized in healthcare. They fulfill diverse purposes, such as diagnosis, continuous monitoring, and tracking patient progress following treatment or drug therapy. Based on the patient’s care needs, these devices can be grouped into two primary categories: inpatient and outpatient applications.
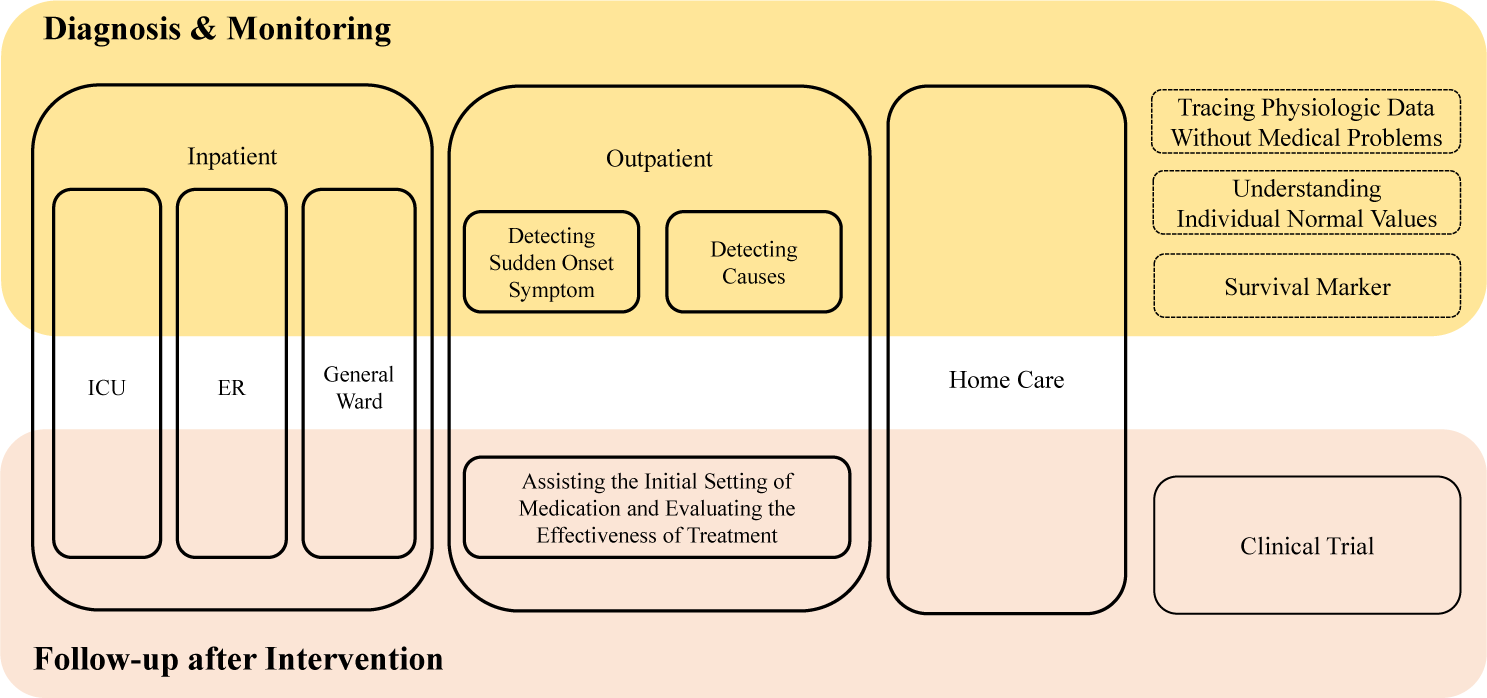
For inpatients, the utility of wearable devices spans various care settings, including general wards, intensive care units, and emergency rooms. Each setting requires tailored functionalities to address the specific needs and challenges inherent to these environments.
Conversely, wearable devices play a pivotal role in outpatient care in detecting conditions associated with sudden-onset symptoms and uncovering the underlying causes of ambiguous symptoms. They are especially valuable during the initiation of drug therapies, where continuous monitoring is essential to ensure both efficacy and safety.
Moreover, wearable devices can be indispensable tools for remote healthcare, empowering patients with home care capabilities. They enable establishing individualized baseline values, such as body temperature and heart rate, and incorporate systems referred to as survival markers. These survival markers are seamlessly integrated with smartphones, allowing for the automatic transmission of critical patient data to medical centers or emergency services when needed.
Additionally, wearable devices excel in monitoring various physiological signals, providing clinicians with valuable insights into patients’ health statuses. They also offer significant advantages in clinical trial settings by enabling continuous, real-world data collection in everyday environments, thereby enhancing the efficiency and effectiveness of research efforts. The author elaborates on the application of wearable devices in detail, using specific examples.
The utilization of wearable devices holds immense potential to revolutionize the monitoring and diagnosis of patients within hospital wards. These innovative technologies offer a novel approach to tracking and assessing the health status of hospitalized individuals continuously. By providing real-time data and actionable insights, wearable devices present an effective solution for enhancing patient care and clinical decision-making. Exploring the applications of wearable devices for the monitoring and diagnosing ward patients is a pivotal endeavor, with the potential to improve patient monitoring processes and support better clinical outcomes (Areia et al., 2021).
Unlike traditional intermittent glucose monitoring methods, continuous glucose monitoring (CGM) provides real-time data on glucose levels, enabling a detailed understanding of glycemic patterns and changes over time (Garg et al., 2024). This capability proves invaluable, particularly in critical scenarios such as diabetic hyperosmolar coma or diabetic ketoacidosis, where timely and precise adjustments to insulin therapy are essential for stabilizing blood sugar levels and preventing life-threatening complications.
Furthermore, integrating CGM into clinical practice equips healthcare providers with actionable insights into patients’ glucose dynamics, enabling them to tailor treatment regimens effectively. Leveraging this data allows clinicians to accurately determine the appropriate dosage and timing of intravenous insulin administration, optimizing glycemic control and minimizing the risk of hypoglycemia or hyperglycemia-related complications.
In parallel, continuous temperature monitoring devices add another dimension to real-time patient assessment (Olson et al., 2023). Fever and febrile symptoms are common manifestations across various medical conditions, ranging from infectious diseases to connective tissue disorders. Accurately identifying the underlying cause of fever can be challenging, particularly in patients with complex medical histories or compromised immune systems. Continuous temperature monitoring provides clinicians with a steady stream of temperature data, facilitating early detection of fever onset, monitoring its progression, and guiding therapeutic interventions (Olson et al., 2023). This proactive approach to temperature management is particularly critical in septic patients, where timely identification and treatment of infections can significantly improve outcomes.
Additionally, electrodermal activity (EDA) monitoring has emerged as a promising tool for assessing neurological function and detecting seizure activity (Poh et al., 2012). Beyond its established role in epilepsy management, EDA monitoring has potential applications in detecting seizures secondary to systemic disturbances, such as electrolyte imbalances, antibiotic toxicity, or metabolic disorders. This approach to seizure detection may enhance diagnostic accuracy and support better clinical outcomes through improved management (Casanovas Ortega et al., 2022).
Similarly, wearable bladder monitoring devices offer a novel solution for managing urinary incontinence and promoting bladder health in patients with reduced mobility (Toymus et al., 2024). These devices utilize non-invasive sensors to continuously monitor bladder volume and predict voiding events, providing caregivers with real-time alerts when the bladder nears fullness. By facilitating timely interventions, such as prompted toileting or diaper changes, these devices help minimize urinary accidents and associated complications, including urinary tract infections and skin breakdown (Toymus et al., 2024).
In rehabilitation medicine, activity-measuring devices provide valuable insights into patients’ physical function and mobility. These devices employ accelerometry and motion-sensing technologies to quantify various aspects of physical activity, such as step count, duration, and intensity. By offering objective measures of activity levels, rehabilitation physicians can assess patients’ progress, track functional outcomes, and tailor interventions to individual needs (Dobkin & Dorsch, 2011). Additionally, integrating these devices with electronic health record systems facilitates seamless data exchange and interdisciplinary collaboration, optimizing care delivery and ensuring continuity of care.
In the ever-evolving healthcare landscape, wearable technology has emerged as a cornerstone in the shift toward personalized and preemptive patient care. Particularly in diagnosing diseases characterized by sudden and transient symptoms, as well as managing chronic conditions prone to abrupt health deterioration, wearable devices offer a promising avenue for both patients and healthcare providers (Dunn et al., 2018). These innovative tools are not merely accessories but vital components in the continuum of care, especially in outpatient management. By enabling real-time, continuous monitoring of physiological data, wearable technology bridges the gap between episodic clinical assessments and the dynamic fluctuations inherent in patient conditions (Pevnick et al., 2016). This facilitates early detection of potential health crises and fosters a nuanced understanding of disease patterns, empowering patients with chronic diseases to lead safer, more informed lives outside the hospital setting (Sim, 2019). Wearable devices thus stand at the forefront of transforming reactive healthcare models into proactive wellness strategies. This chapter explores their utility in outpatient care through illustrative examples, highlighting their transformative impact on patient management and healthcare outcomes.
Wearable devices are revolutionizing the diagnosis of conditions with sporadic symptoms, offering an innovative approach to capturing elusive health data. These devices provide critical insights into intermittent health issues by detecting physiological biomarkers, enabling early and accurate diagnoses.
Many heart-related conditions are difficult to diagnose if not assessed immediately when symptoms occur. For this reason, tools that provide real-time access to physiological biomarkers during symptomatic episodes are essential. Smartwatches, for instance, can detect irregular heart rhythms, serving as practical tools for identifying potential arrhythmias by correlating patient-reported symptoms with heart activity (Perez et al., 2019). Their capability to record electrocardiograms during symptomatic periods helps differentiate psychological phenomena from physiological causes of tachycardia, thereby improving diagnostic accuracy (Caillol et al., 2021). Blood pressure monitoring serves as an essential diagnostic tool in assessing patients who experience intermittent syncope, a condition characterized by sudden, temporary losses of consciousness due to various causes such as vasovagal syncope, orthostatic hypotension, temporary arrhythmias from cardiovascular dysfunction, and even epilepsy (Groppelli et al., 2022). Understanding whether a drop in blood pressure precipitates a syncope episode or if it coincides with arrhythmias is invaluable, particularly during initial patient assessments. This insight helps delineate the cardiovascular contributions to syncope, offering a clearer picture of the episode’s etiology (Groppelli et al., 2022).
Identifying whether a syncope episode results from a drop in blood pressure only or coincides with arrhythmias provides invaluable insight, particularly during initial patient assessments. This understanding clarifies the cardiovascular contributions to syncope, offering a more precise view of the episode’s etiology. To further refine the diagnostic process for syncope, integrating EDA measurements provides a non-invasive and highly sensitive approach (Poh et al., 2010). EDA enhances clinicians’ ability to assess the autonomic nervous system’s role in syncope, offering complementary data to blood pressure and cardiac rhythm analyses. The incorporation of EDA measurement into diagnostic workflows enriches understanding of the complex interplay between physiological systems involved in syncope (Poh et al., 2010). By capturing nuanced autonomic signatures associated with different types of syncope, clinicians can more effectively differentiate between neurogenic and cardiac causes (Brignole et al., 2018). This comprehensive approach not only improves the differential diagnosis of syncope but also equips healthcare professionals with a broader set of tools (Brignole et al., 2018). It enables the development of more targeted and effective management strategies, paving the way for tailored treatments that address the specific underlying causes of syncope (Fedorowski et al., 2023). This ultimately enhances patient care and clinical outcomes.
Wearable devices are revolutionizing healthcare by offering advanced, continuous monitoring of various health metrics. This real-time data collection is especially valuable for managing chronic conditions, as it provides detailed insights that help doctors make more accurate diagnoses and treatment decisions.
For instance, individuals who experience elevated blood pressure only in clinical settings, a condition known as white coat syndrome, can benefit from wearable devices that track blood pressure throughout the day. This allows for a more accurate assessment of hypertension (Parati et al., 2014). By continuously providing detailed health information, wearable devices support personalized treatment strategies and proactive healthcare management.
In the complex landscape of medical conditions that challenge modern healthcare, a distinct group of diseases stands out due to their sudden onset and their profound impact on patients’ lives. Conditions such as epilepsy, narcolepsy, arrhythmia, and hypoglycemia—particularly prevalent among individuals diagnosed with Type I diabetes—constitute a category of chronic intractable diseases (Dauvilliers et al., 2007; Kirchhof et al., 2016; Martyn-Nemeth et al., 2017). These conditions share a common tendency to trigger acute pathological events without warning, resulting in rapid and significant health deterioration (Cryer, 2008; Fisher et al., 2014; Hindricks et al., 2021; Thorpy & Krieger, 2014). Such episodes can severely affect a patient’s quality of life and, in extreme cases, become life-threatening (Quintas et al., 2012; Raggi et al., 2019; Seaquist et al., 2013; Thrall et al., 2006).
The advent of predictive monitoring devices marks a significant advancement in the management of diseases characterized by sudden and transient symptoms. These technologies provide a critical window of opportunity for intervention before an acute episode fully manifests, potentially drastically reducing the risk of severe health deterioration.
For individuals with Type I diabetes, devices capable of predicting hypoglycemic events can prompt timely interventions, such as consuming carbohydrates or adjusting insulin dosages preemptively. This proactive approach significantly reduces the risk of severe hypoglycemia and its associated complications (Cryer, 2008). The fear of hypoglycemia has been shown to influence glycemic variability and self-management behavior in young adults with Type 1 diabetes, highlighting the importance of predictive monitoring in improving patient outcomes and quality of life (Martyn-Nemeth et al., 2017).
CGM systems have demonstrated effectiveness in reducing time spent in hypoglycemia while simultaneously decreasing Hemoglobin A1C levels in both children and adults with Type 1 diabetes (Battelino et al., 2012). These systems provide real-time glucose data and trend information, allowing for more informed decision-making and timely interventions. The integration of CGM with predictive algorithms can further enhance the management of Type 1 diabetes by forecasting glucose levels and alerting patients to potential hypoglycemic events before they occur (Sparacino et al., 2007).
Leveraging EDA as a predictive tool offers a sophisticated approach to forecasting seizure episodes in epilepsy patients. By enabling the early detection of imminent seizures, EDA monitoring provides individuals with the critical opportunity to position themselves in a secure setting before an episode, thereby substantially reducing the potential for harm and enhancing overall safety (Regalia et al., 2019). Recent advancements in wearable technology have made it possible to monitor EDA and other physiological parameters in ambulatory settings continuously. Regalia et al. (2019) demonstrated the feasibility of using wrist-worn devices to detect seizures and predict seizure risk in patients with epilepsy. The study showed that machine learning algorithms applied to multimodal physiological data, including EDA, could achieve seizure detection performance significantly better than chance for most patients studied.
In the realm of outpatient care, wearable devices have become an indispensable tool for assessing the effectiveness of prescribed medications. These advanced technologies facilitate continuous monitoring of vital biometrics, including blood glucose, blood pressure, and heart rate, offering a comprehensive and dynamic overview of a patient’s response to treatment.
Diagnosing Type 2 diabetes with intricate glycemic control challenges presents physicians with the critical task of selecting the most suitable treatment from a diverse range of therapeutic options (ElSayed et al., 2023). Following the initiation of medication, continuous blood glucose monitoring provides invaluable data to guide necessary adjustments to the treatment plan (Battelino et al., 2019). This detailed oversight is essential for stabilizing glucose levels and mitigating the risks associated with hyperglycemia and hypoglycemia (Danne et al., 2017).
As the patient’s condition stabilizes and glucose levels, along with Hemoglobin A1C readings, fall within acceptable ranges, the intensive monitoring required during the initial stages can be scaled back. Transitioning to regular, scheduled blood sampling for long-term follow-up represents a strategic shift in management, from continuous real-time monitoring to periodic assessments. This adaptive strategy ensures ongoing vigilance over the patient’s glycemic status while balancing effectiveness with patient comfort and convenience. It underscores the dynamic nature of diabetes care and highlights wearable technology’s integral role in facilitating personalized treatment plans.
Similar to how CGM has revolutionized diabetes management by enabling real-time tracking of blood sugar levels, continuous blood pressure monitoring devices have transformed hypertension care (Watanabe et al., 2017). These devices allow for the dynamic observation of blood pressure throughout the day, offering invaluable insights into the effectiveness of antihypertensive medications (Kario et al., 2019). By providing a detailed profile of blood pressure fluctuations in response to treatment, continuous blood pressure monitoring facilitates precise adjustments to medication dosages, ensuring optimal hypertension control (Stergiou et al., 2021). This approach enhances the personalization of care, empowering both patients and healthcare providers with the data required to make informed decisions about treatment strategies (Kario et al., 2019). Ultimately, this technology improves patient outcomes and quality of life.
DFree, an innovative device designed to monitor bladder volume, represents a cutting-edge tool for managing neurogenic bladder conditions (Hofstetter et al., 2022). Providing precise, real-time data on bladder fullness enables urologists to gain deeper insights into bladder functionality, offering a clearer understanding of a patient’s condition beyond subjective symptom reports (Hafid et al., 2023). Integrating DFree into clinical practice facilitates a nuanced understanding of neurogenic bladder symptoms and provides a robust framework for evaluating the efficacy of medications (Hofstetter et al., 2022).
This objective approach allows urologists to correlate the device’s quantitative readings with patient-reported symptoms, creating a comprehensive picture of the treatment’s impact on bladder control and functionality (Hafid et al., 2023). As a result, DFree plays a critical role in tailoring individualized treatment plans, ensuring each patient receives the most effective interventions based on empirical data (Hofstetter et al., 2022).
Moreover, DFree offers the additional benefit of mitigating the placebo effect, a common challenge in treatment evaluations. Delivering tangible, quantifiable data on bladder volume before and after medication administration distinguishes actual physiological improvements from perceived effects due to placebo influence (Hafid et al., 2023). This capability is invaluable for the scientific assessment of therapeutic outcomes, ensuring that clinical decisions are grounded in objective evidence rather than subjective interpretation (Hofstetter et al., 2022).
As patients experience symptom relief and achieve a stable condition, reliance on DFree for continuous monitoring can be reduced. However, its role in the initial stages of treatment, particularly in distinguishing genuine therapeutic benefits from placebo effects, highlights its critical importance in both the management and understanding of neurogenic bladder conditions (Hafid et al., 2023).
The Sleep Cycle app is a sophisticated smartphone application that employs advanced algorithms to monitor sleep by detecting user movements and sounds throughout the night” (Adjust, 2023; Apple, n.d.). This technology serves as a complementary tool to traditional polysomnography, offering clinicians valuable insights into patients’ sleep patterns in their natural environment. The real-world data captured by the Sleep Cycle enhances the understanding of individual sleep behaviors, enabling the development of personalized treatment strategies.
Similarly, the Oura Ring (Svensson et al., 2024) extends sleep analysis capabilities beyond clinical settings. This wearable device meticulously analyzes sleep stages, generating comprehensive reports on sleep quality directly from the comfort of patients’ homes. The Oura Ring’s convenience and accuracy make it an invaluable asset for monitoring sleep health and evaluating the impact of interventions such as sleep apnea surgery, pharmacological treatments, or lifestyle adjustments on sleep quality. Together, the Sleep Cycle and the Oura Ring represent the forefront of sleep-monitoring technology. These tools provide both patients and healthcare professionals with accessible and accurate means of evaluating sleep health and tailoring interventions to achieve optimal sleep patterns (Beattie et al., 2017).
Orthopedic surgeons and rehabilitation medicine specialists can significantly enhance patient care protocols by integrating activity trackers into postoperative and injury recovery processes (Szeto et al., 2023). These advanced devices provide comprehensive data beyond conventional symptom reporting, offering detailed insights into patients’ physical activity levels and recovery progress (Natarajan et al., 2023).
Monitoring a patient’s mobility and activity patterns is invaluable in the critical stages following surgery or injury. Activity trackers meet this need by providing continuous, objective data on a range of motion, step count, and overall physical engagement (van Dijk-Huisman et al., 2020). This data enables medical professionals to precisely tailor rehabilitation programs, adjusting exercises and therapy sessions based on real-time feedback (Thijs et al., 2019).
Activity trackers also play a pivotal role in motivating patients during their recovery journey. By setting achievable activity goals and tracking progress, these devices encourage patients to adhere to their rehabilitation plans, fostering active participation in their recovery (Ummels et al., 2021). This not only accelerates the healing process but also empowers patients with a sense of control and accomplishment (Kelly et al., 2021).
In the treatment and follow-up phases, insights from activity trackers significantly influence clinical decision-making. Orthopedic surgeons and rehabilitation specialists can use this data to assess treatment effectiveness, make informed adjustments, and deliver personalized care aligned with each patient’s unique recovery trajectory (Daskivich et al., 2019).
The adoption of activity trackers in orthopedic and rehabilitation medicine signifies a shift toward a more dynamic, data-driven approach to patient recovery. By leveraging the detailed activity data these devices provide, healthcare professionals can optimize recovery outcomes, ensuring patients regain their daily activities with confidence and resilience (Tazreean et al., 2022).
Home care represents a specialized remote care system distinct from outpatient care, designed to support individuals who face challenges visiting hospitals due to mobility issues or geographical barriers. This mode of care is particularly vital for the elderly, who often find it difficult to travel to and from medical facilities. The advancement and integration of wearable devices are pivotal in enhancing home care services. These devices, equipped to measure and transmit biometric data in real-time, enable healthcare professionals to monitor and assess patients’ health remotely. The continuous flow of accurate and up-to-date data ensures that medical advice and interventions are well-informed, significantly improving the quality and effectiveness of home care (Majumder et al., 2017).
Innovations in home care also include the development of robotic caregiving systems. These systems maintain a constant data link among wearable devices, robots, and medical centers, ensuring uninterrupted care even in cases where connectivity to medical facilities is compromised (Stavropoulos et al., 2020). Robotic systems utilize short-range communication technologies to interface directly with wearable devices, allowing them to collect and analyze real-time biological data, such as heart rate, blood pressure, oxygen levels, and other vital signs, without requiring an active internet connection to the medical center.
This autonomous functionality enables robotic systems to respond immediately to patients’ needs. For instance, when deviations in vital signs occur, these intelligent systems can execute predefined actions, such as administering medications as per the care plan, performing basic first-aid procedures, or alerting emergency services and the healthcare team via alternative communication channels once connectivity is restored (Pham et al., 2018).
Such capabilities are particularly beneficial for patients living alone or in remote areas where immediate human medical intervention may not be readily available. Continuous monitoring and care enhance patient safety, improve health outcomes, and provide peace of mind for both patients and their families (Abdi et al., 2018). This integration of wearable devices and robotic caregiving systems creates a more resilient and adaptable home care framework capable of addressing the complex needs of patients outside traditional medical settings (Stavropoulos et al., 2020).
Wearable devices have emerged as transformative tools in capturing real-world biometric parameters, revolutionizing clinical trial research. The study by Birkeland et al. highlights the potential of wearable digital technologies to provide fresh perspectives and insights into the nuances of clinical investigations (Birkeland et al., 2017). In their pioneering research, the Fitbit Flex, a consumer-grade wearable device, was employed to measure step counts as a practical metric for evaluating the impact of late sodium channel inhibition (specifically, the drug ranolazine) on participants’ daily activities. Their findings revealed a nuanced relationship between late sodium channel inhibition and physical activity levels, demonstrated by an overall decrease in step counts within the study cohort.
The application of wearable devices in Birkeland et al.’s research exemplifies the integration of real-world data with traditional laboratory findings, enriching the depth and reliability of clinical trials. By combining activity metrics with clinical data, researchers can better understand a drug’s effects in controlled environments and the variable conditions of daily life (Izmailova et al., 2018). This approach not only broadens the scope of clinical research but also enhances its applicability to patient care, providing a more holistic perspective on treatment efficacy and patient outcomes.
These advancements signal a promising future for clinical trials, where wearable technologies can generate richer, more nuanced insights into medical interventions. The integration of real-world data captured through wearables refines the precision of clinical research, ensuring it better reflects actual patient experiences and improves the overall quality and reliability of trial outcomes (Coravos et al., 2019).
The incorporation of wearable devices into clinical trials offers several multifaceted advantages. First, the immediate adoption of existing wearable technologies accelerates the research process, enabling rapid exploration of their applications within clinical trial frameworks (Goldsack et al., 2020). Second, the investigative nature of clinical trials often uncovers new domains for wearable device applications, expanding their utility beyond original expectations.
Moreover, while the primary focus of clinical trials may not always involve developing new wearable devices, such innovation often emerges as a valuable byproduct. These newly developed devices can enhance clinical care and treatment methodologies, further enriching healthcare practices (Dunn et al., 2018). Thus, clinical trials employing wearable technologies act as catalysts for technological advancement by identifying new indications for existing devices and stimulating the creation of novel wearables.
This dynamic interplay between clinical research and wearable technology underscores the pivotal role of clinical trials in fostering innovation. By aligning the evolution of wearable devices with the practical needs and challenges of healthcare, these trials enhance the scope and effectiveness of patient monitoring and treatment strategies.
In specific contexts, precise monitoring of physiological data is critically important, particularly for vulnerable populations such as infants and toddlers. These young individuals are at heightened risk for rapid-onset conditions like fevers and potentially life-threatening issues such as sleep apnea (Airaksinen et al., 2020). Continuous tracking of physiological changes and bodily positions, whether supine, prone, or in a decubitus position, can be invaluable in ensuring their safety and well-being (Airaksinen et al., 2020). Devices equipped with position-detecting sensors provide parents and caregivers with real-time insights into a child’s sleeping posture, mitigating risks associated with certain positions (Airaksinen et al., 2020). For example, monitoring the supine position, recommended to reduce the risk of Sudden Infant Death Syndrome, underscores the importance of such devices.
Additionally, devices capable of monitoring a baby’s breathing rate add a crucial layer of safety by alerting caregivers to potential respiratory issues that may require immediate attention. These tools not only offer peace of mind to parents but also serve an essential role in the early detection and prevention of health complications.
Another scenario requiring stringent physiological monitoring involves individuals in extreme environments, such as high-altitude locations, underwater settings, or space (Castiglioni et al., 2022). In these conditions, the body experiences significant stress, making it vital to monitor parameters such as heart rate, oxygen saturation, and body temperature (Castiglioni et al., 2022). For instance, at high altitudes, the risk of hypoxia caused by reduced oxygen levels poses a serious threat. Wearable devices designed to function reliably in such environments and deliver real-time data can be life-saving by enabling immediate responses to physiological changes and ensuring the safety of those exposed to these harsh conditions (Castiglioni et al., 2022).
The integration of wearable technology for physiological monitoring in these scenarios illustrates its profound potential to improve health outcomes. By providing continuous, detailed data, these devices empower caregivers and individuals to make informed decisions regarding health and safety. This development exemplifies the evolving intersection of technology and healthcare, enhancing the quality of care in both routine and extreme conditions.
Individual physiological variations mean that what constitutes a ‘normal’ value for one person may differ for another. This is particularly evident in body temperature, a vital sign that varies among individuals (Obermeyer et al., 2017). As a result, a person may experience symptoms associated with fever, such as warmth and chills, even if their temperature does not exceed the conventional fever threshold. These subjective experiences highlight the importance of personalized health monitoring (Ley et al., 2023).
Wearable devices designed to monitor body temperature provide a sophisticated solution to this challenge. By continuously tracking an individual’s temperature over time, these devices can establish a personalized baseline temperature during periods of good health (Majumder et al., 2017). This baseline serves as a reference point, reflecting the user’s normal temperature range in the absence of illness.
With access to this personalized data, individuals can promptly detect deviations from their baseline temperature range. Such deviations may signal the onset of fever or other health issues, even when the readings do not meet the general fever criteria (Smarr et al., 2020). This real-time insight into one’s physiological state enables timely action, whether it involves seeking medical advice or monitoring for additional symptoms.
This personalized approach to health management empowers individuals to better understand their bodies and respond more effectively to potential health changes. It represents a shift toward more individualized healthcare, where interventions are precisely tailored to meet each person’s unique needs (Coravos et al., 2019).
In the dynamic and high-risk environments of modern warfare, ensuring soldiers’ safety and operational efficiency is paramount. Wearable devices capable of monitoring vital signs, such as pulse rate and oxygen saturation, combined with telecommunication capabilities, represent a significant advancement in battlefield technology (Friedl, 2018). These devices enable real-time biologic status monitoring, offering critical support in both combat and rescue operations. For individual soldiers, particularly infantry on the front lines, wearable devices equipped with pulse and oxygen saturation sensors can be a lifeline. In scenarios where a soldier is wounded or separated from their unit, traditional rescue efforts are often hindered by the absence of immediate information regarding the soldier’s condition and location. Wearable technology provides field commands with real-time updates on a soldier’s status, allowing them to ascertain whether the individual is alive and needs urgent medical intervention (Friedl, 2018). This capability dramatically enhances the speed and precision of rescue operations, potentially saving lives through timely care.
Additionally, the telecommunication functionality of these devices ensures that biological data is continually transmitted to command centers, even amidst the chaos of battle or in cases of separation. This constant stream of information enables dynamic decision-making and resource allocation, tailoring rescue missions to the specific needs of injured personnel. As combat gear becomes increasingly electronically integrated, the incorporation of wearable vital sign monitors into soldiers’ equipment is both feasible and expected (Friedl, 2018). These compact, efficient devices align with the trajectory of modern warfare, where soldiers already carry advanced electronic systems alongside traditional weaponry and survival kits.
In Manned-Unmanned Teaming (MUM-T) operations, where human leaders coordinate robotic systems on the battlefield, the stakes are particularly high (Ryan, 2018). The operational effectiveness of these missions hinges on the health status of human field commanders, whose incapacitation could lead to delays, miscommunications, or even catastrophic failures. In the worst-case scenario, an incapacitated commander unable to transfer leadership could jeopardize the mission and lives. Wearable devices equipped with vital sign sensors and telecommunication features provide a continuous stream of data on the field commander’s physical condition (Friedl, 2018). This allows command centers to monitor the leader’s status in real-time and, if necessary, reassign leadership roles dynamically. Such capabilities ensure seamless operational continuity, as robotic units within the MUM-T framework can adapt to directives from new leaders designated by the command center (Ryan, 2018). These autonomous or semi-autonomous units can also be instructed to abort the mission and return to base if circumstances necessitate.
This integration of wearable technology enhances the resilience of MUM-T operations by ensuring human-machine collaboration remains efficient and adaptable, even under adverse conditions (Ryan, 2018). Enabling immediate responses to health issues safeguards human commanders’ well-being while maintaining the mission’s operational integrity. The potential applications of this technology extend well beyond military operations. Rescue teams working in disaster zones or extreme terrains, as well as extreme sports enthusiasts in remote or hazardous environments, could greatly benefit from such systems. Wearable devices can enhance the safety and efficiency of search and rescue missions by enabling precise tracking of team members and providing real-time vital sign monitoring. This ensures rapid responses to emergencies and improves coordination in high-stress scenarios. Similarly, for individuals engaging in high-risk activities, wearable devices that monitor vital signs and include global positioning system (GPS), functionality can be life-saving (Friedl, 2018). These devices provide critical information in emergencies that facilitates timely interventions and rescue efforts.
The deployment of wearable devices with vital sign monitoring and telecommunication capabilities offers a forward-thinking solution to longstanding challenges in high-risk environments. In military operations, such technology ensures the continuous monitoring and locability of personnel, improving the effectiveness of rescue missions and safeguarding lives (Friedl, 2018). Beyond the battlefield, its applications in disaster response and extreme sports highlight its potential to enhance safety and resilience in hazardous conditions. This wearable technology integration revolutionizes operational strategies and underscores the evolving synergy between technology and human safety in dynamic and unpredictable environments.
REVOLUTIONIZING REMOTE HEALTHCARE AND BEYOND
The rapid advancement of smartphone technology and its peripherals has catalyzed the development of wearable devices, positioning them as transformative tools across various sectors, particularly in healthcare. Despite initial enthusiasm, many wearable devices face high abandonment rates within the first year of use, revealing a disconnect between consumer expectations and device utility. This trend highlights the urgent need for wearable devices that are not only technologically advanced but also practical, sustainable, and closely aligned with user needs.
Wearable devices have demonstrated immense potential in healthcare, enabling continuous monitoring of vital signs and aiding in the diagnosis and management of chronic conditions. For inpatients, these devices provide real-time data on critical metrics such as glucose levels, body temperature, and seizure activity, enhancing patient care and clinical decision-making. In outpatient care, they have proven invaluable for diagnosing conditions with intermittent symptoms, such as arrhythmias and syncope, and for managing chronic diseases by monitoring health markers and predicting potential emergencies.
Beyond traditional healthcare settings, wearable devices excel in military operations, rescue missions, and extreme environments. On the battlefield, these devices serve as critical tools for monitoring the biological status of personnel, enabling real-time health updates imperative for dynamic decision-making and resource allocation. Similarly, in rescue missions and extreme environments, wearables provide real-time monitoring of individuals exposed to challenging conditions, offering personalized health insights and enabling timely interventions.
The future of wearable technology lies in its ability to enable simple, compact devices to capture vital signs and other biometric indicators with precision. By leveraging encrypted information networks, these real-time data streams can be securely transmitted to telemedicine centers for continuous analysis. This integration would allow healthcare providers to monitor patients remotely, identify emerging health risks, and respond promptly, making effective home care a tangible reality.
Achieving this vision requires transdisciplinary research that brings together healthcare professionals, engineers, data scientists, and policymakers. Such collaborations can address the technological, ethical, and logistical challenges associated with wearable devices, ensuring they are seamlessly integrated into healthcare systems and other high-stakes settings. By pushing the boundaries of innovation, wearable technology can redefine the possibilities of remote healthcare and home care, offering personalized, efficient, and sustainable solutions that enhance health outcomes and operational safety.






